Using an alternate method decreases the demand over a purely natural useful resource and might help satisfy offer chain sustainability initiatives. They may be getting use across the globe as corporations look for to cut back their reliance on organic assets.
LPS are the most crucial parts on the cell wall or mobile membrane of Gram negative bacteria; and they're typically pyrogenic in character. They can be really warmth-stable in mother nature and as a consequence usually are not simply destroyed under typical sterilization conditions. Bacterial endotoxins or LPS are ubiquitous in character and are available in the air, water, inside the laboratory and even at perform environments.
Irrespective of their sensitivity and specificity for endotoxins of Gram adverse germs, the LAL test is proscribed – because it can not detect exotoxins of Gram constructive micro organism and cellular factors of viruses and fungi.
Publishing on IntechOpen will allow authors to gain citations and locate new collaborators, meaning more people see your get the job done not merely from the very own area of analyze, but from other linked fields also.
The animals has to be set from the boxes one hour before the test and continue being in them all through the test. Make sure that the place temperature where the test is carried out is within just three° of that in the rabbits dwelling quarters or wherein the rabbits are already held for eighteen hrs ahead of the test. Withhold food items from your animals right away and right up until the test is completed; withhold drinking water throughout the test.
At the present time delivers a virtual occasion in individual to the horseshoe crab sanctuary Beach front, where by we assistance conserve horseshoe crabs stranded from spawning activities.
You can find also some proof that phosphate-containing formulations can also be influenced by LER. Having said that, the LER effect has only been observed together formulations from the aforementioned excipients, and never in specific Uncooked elements.
Consequently, enhanced tactics can be Specially helpful in demasking and capturing LPS molecules in circulating blood. Therefore, new strategies is likely to be handy in detecting the presence of trace quantities of endotoxin while in the blood and correctly analyzing the clinical effect of immediate hemoperfusion as well as the therapeutic potential of recent drug candidates, including anti-endotoxin brokers and antimicrobial peptides [84].
Over the LER phenomenon, a common formulation matrix that contains sodium citrate and polysorbate in biopharmaceuticals brings about the inability to Get well LPS in a time-dependent way when spiked into undiluted samples [19,twenty]. It's not solely very clear whether masked endotoxins are biologically active in vivo, While masked endotoxin is reported to get a strong result in of immune responses [21]. It truly is attention-grabbing to notice that a similar phenomenon in clinical specimens is click here assumed to arise all through disaggregated LPS–HDL binding, As an illustration [22].
However, while in the interest of assuring the caliber of injection preparations since they are actually administered, the following nondestructive tests are delivered for demonstrating the suitability of constituted answers when they're organized just before use.
Big- AND Smaller-Quantity INJECTIONS In which applied Within this Pharmacopeia, the designation Substantial-quantity intravenous Resolution applies to only one-dose injection that is meant for intravenous use and is particularly packaged in containers labeled as made up of a lot more than a hundred mL.
Pharmaguideline is actually a pharmaceutical site the place pharmaceutical ideas are described in very simple and simply easy to understand language for gurus and pupils. All content and SOPs are prepared by Ankur Choudhary.
Gel Clot assay can be a qualitative LAL test for detection of Gram-negative micro organism endotoxins. The Gel Clot assay is operate in tubes which can be put in a drinking water tub or in dry heated oven at 37°C. Following a 1-hour incubation time period, the tubes are flipped a hundred and eighty°. A firm clot that stays in the bottom on the tube implies a good reaction. If your liquid flows down the facet of the tube, the result is adverse for endotoxins.
What on earth is Open up Access? Open Entry read more is surely an initiative that aims to help make scientific study freely available to all. So far our community has made over one hundred million downloads. It’s based upon concepts of collaboration, unobstructed discovery, and, most importantly, scientific progression.
 Patrick Renna Then & Now!
Patrick Renna Then & Now! Richard "Little Hercules" Sandrak Then & Now!
Richard "Little Hercules" Sandrak Then & Now! Marques Houston Then & Now!
Marques Houston Then & Now!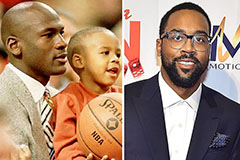 Marcus Jordan Then & Now!
Marcus Jordan Then & Now! Stephen Hawking Then & Now!
Stephen Hawking Then & Now!